Insight
Blood plasma fractionation: Engineering and facility design for the future of biotherapeutics

seraficus
Insight
seraficus

Here’s something you might not know: Over 90% of global plasma is processed in just a handful of countries. Developing local fractionation capacity is not only a healthcare priority but also a matter of national biosecurity.
In the rapidly evolving biopharmaceutical landscape, human blood plasma is a critical raw material for lifesaving therapies. Plasma-derived medicinal products (PDMPs) such as immunoglobulins, albumin, and clotting factors are essential for treating immunodeficiencies, bleeding disorders, and trauma. However, the journey from donor to drug involves a highly complex, regulated, and engineering-intensive process.
As an engineering consultancy specializing in life sciences, we play a pivotal role in designing and optimizing plasma fractionation facilities that meet stringent regulatory standards while ensuring operational efficiency, scalability, and sustainability. This article explores the science, engineering, and infrastructure behind plasma fractionation and purification, with a focus on modern facility design and process integration.
The origins of plasma fractionation trace back to World War II, when the need for stable, transportable blood products led to the development of the Cohn cold ethanol fractionation process. Dr. Edwin Cohn’s method separated plasma proteins by manipulating ethanol concentration, pH, and temperature, enabling the isolation of therapeutically valuable fractions.
This innovation laid the foundation for today’s plasma-derived therapies and continues to influence modern bioprocessing strategies.
These biologics are not synthetically manufactured; they must be extracted from human plasma, making the engineering of fractionation facilities a matter of both public health and national resilience.
The Cohn process remains the industry standard for initial plasma fractionation. It involves sequential protein precipitation by adjusting:
Each step isolates a specific protein fraction:
The process requires precise control of thermodynamic parameters and robust containment to handle flammable solvents.
This process targets Factor VIII and involves:
Cryoprecipitate is rich in fibrinogen, von Willebrand factor, and Factor VIII, making it vital for hemophilia treatment.
Chromatographic methodologies, introduced into plasma fractionation workflows in the early 1980s, have significantly enhanced the recovery and purity of therapeutic plasma proteins compared to traditional ethanol-based fractionation methods such as the Cohn process.
These techniques exploit the physicochemical properties of plasma proteins — such as size, charge, and binding affinity — to achieve high-resolution separation under controlled conditions.
The hybrid plasma fractionation process combines the traditional Cohn cold ethanol fractionation with modern chromatographic purification techniques to enhance the quality, safety, and yield of plasma-derived products.
To meet modern purity and safety standards, hybrid processes integrate:
These steps are essential for producing sterile, high-purity biologics. This integrated approach enables higher purity, better viral safety, and greater process flexibility compliant with EMA and FDA guidelines, making it the preferred method in modern plasma fractionation facilities.
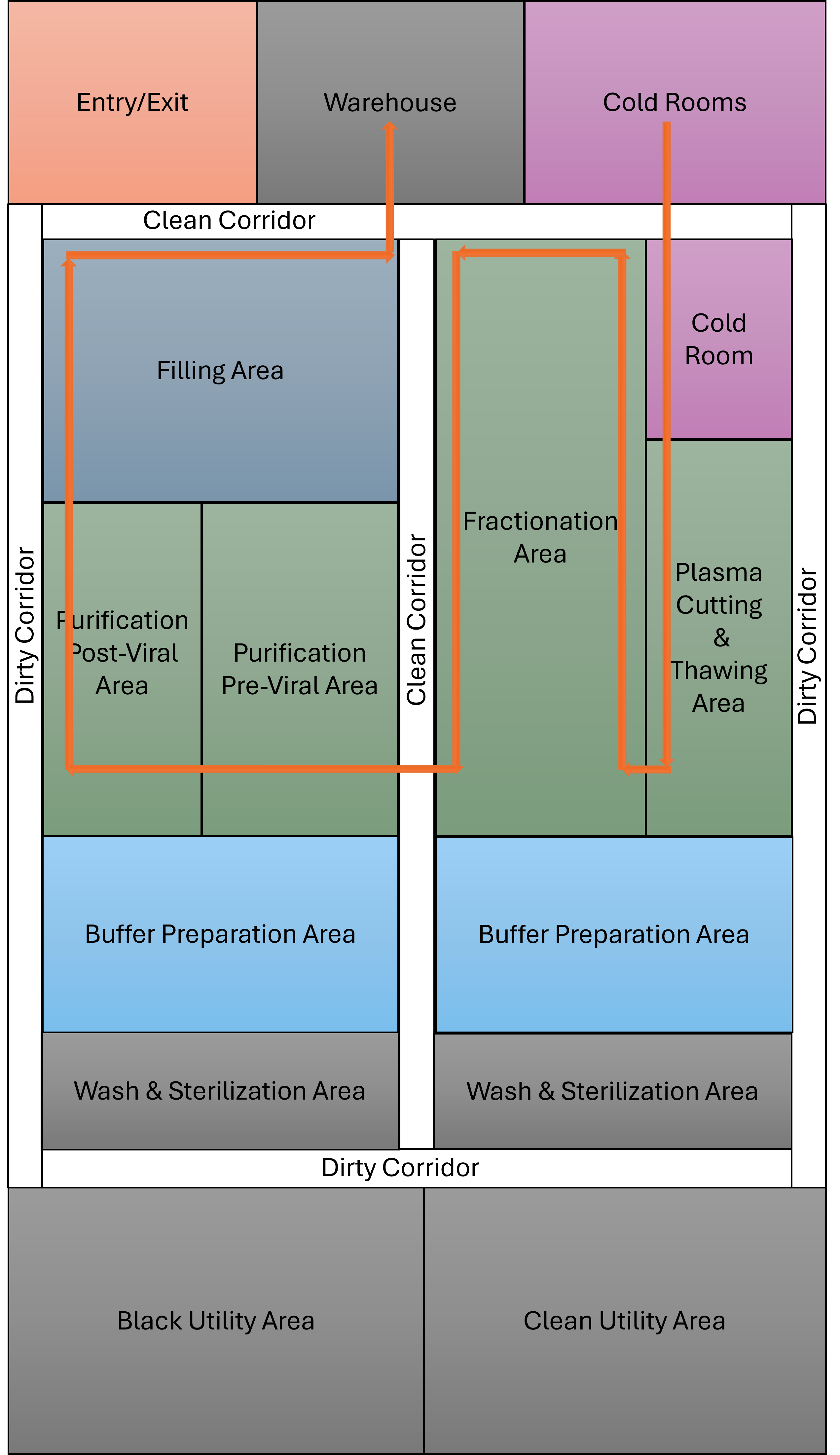
Designing a plasma fractionation facility involves balancing regulatory compliance, process efficiency, and scalability. Key considerations include implementing:
Choosing the right facility design — whether conventional stainless steel, single-use, or hybrid modular — is essential for optimizing production efficiency, scalability, and responsiveness to market demands. Aligning the design with your operational goals and product portfolio ensures long-term success and agility in a competitive biopharmaceutical landscape.
Plasma must be stored at minus 30 degrees C throughout the supply chain, and cold rooms typically occupy 50% of warehouse space. Redundant refrigeration systems and temperature monitoring are critical.
This includes:
Engineering firms must specify and integrate equipment that meets GMP, biosafety, and automation requirements, including the following:
All equipment must be CIP/SIP capable, validated, and integrated into DSC/MES systems for traceability.
Smart manufacturing is transforming plasma processing:
These technologies enhance regulatory compliance, data integrity, and operational efficiency.
As environmental regulations tighten, sustainable design is imperative. Sustainability is not only a regulatory requirement but also a driver of long-term cost efficiency. Key processes to achieve this include:
The plasma fractionation industry is led by a few multinational players:
These companies operate state-of-the-art facilities with capacities ranging from 100,000 to over 1 million liters/year.
According to Fortune Business Insights, the 2025 plasma fractionation market size is estimated at $37+ billion, and it is projected to grow to $60 billion by 2030, a CAGR of about 9%. The top markets worldwide are the U.S., the EU5 (U.K., Germany, France, Spain, and Italy), China, and Japan.
Blood plasma fractionation is a mission-critical domain where engineering excellence directly impacts patient outcomes. Fractionation facilities must be compliant, efficient, and future-ready to ensure the pharmaceutical industry can continue to offer lifesaving solutions.



